| Lattice System |
Possible Variations |
Axial Distances (edge lengths) |
Axial Angles |
Examples |
|---|
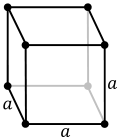
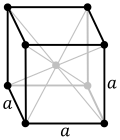
| Cubic |
Primitive, Body-centred, Face-centred |
a = b = c |
α = β = γ = 90° |
NaCl, Zinc Blende, Cu |
| Tetragonal |
Primitive, Body-centred |
a = b ≠ c |
α = β = γ = 90° |
White tin, SnO2, TiO2, CaSO4 |
| Orthorhombic |
Primitive, Body-centred, Face-centred, Base-centred |
a ≠ b ≠ c |
α = β = γ = 90° |
Rhombic sulphur, KNO3, BaSO4 |
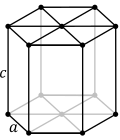
| Hexagonal |
Primitive |
a = b ≠ c |
α = β = 90°, γ = 120° |
Graphite, ZnO, CdS |
| Rhombohedral |
Primitive |
a = b = c |
α = β = γ ≠ 90° |
Calcite (CaCO3, Cinnabar (HgS) |
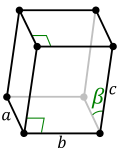
| Monoclinic |
Primitive, Base-centred |
a ≠ b ≠ c |
α = γ = 90°, β ≠ 90° |
Monoclinic sulphur, Na2SO4.10H2O |
| Triclinic |
Primitive |
a ≠ b ≠ c |
α ≠ β ≠ γ ≠ 90° |
K2Cr2O7, CuSO4.5H2O, H3BO3 |













No comments:
Post a Comment